Research
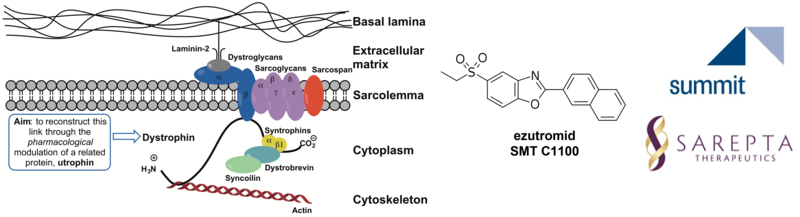
Utrophin Modulators for Duchenne Muscular Dystrophy (with Kay Davies, Physiology, Anatomy and Genetics & Matthew Wood, Institute for Developmental and Regenerative Medicine)
It has been shown that utrophin, the autosomal homologue of dystrophin, can compensate for the lack of functional protein in Duchenne Muscular Dystrophy (DMD). In collaboration with Professor Dame Kay Davies, we developed a screen and discovered the first small molecules capable of upregulating utrophin protein expression in muscle cells. This early work led to a spin-out company (now Summit Therapeutics) and the development of ezutromid. Summit, in partnership with Angela’s group, progressed ezutromid into a Phase 2 clinical study for the treatment of DMD in 2017. Ezutromid showed promising efficacy and evidence of utrophin target engagement after 6 months, but these effects were not seen at 12 months, and ezutromid failed to meet its primary and secondary endpoints. At the time, ezutromid’s target and molecular mechanism were not known and development had to be discontinued.
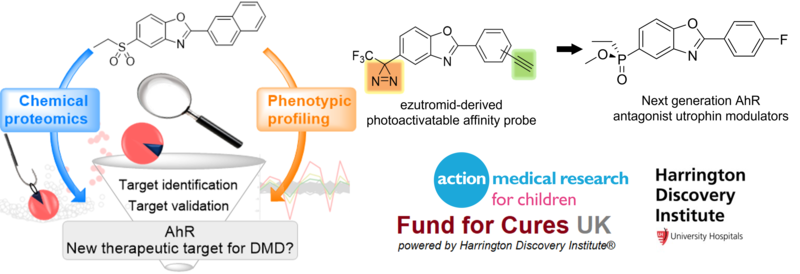
Our group was successful in dissecting ezutromid’s metabolism and mechanism of action, thereby explaining the lack of sustained efficacy, despite initial positive results. We demonstrated that ezutromid binds to the arylhydrocarbon receptor (AhR) with high affinity, and this antagonism of AhR by ezutromid leads to utrophin upregulation, thereby confirming AhR as a viable target for utrophin functional replacement therapies. We have since followed up with a new series of AhR antagonist, utrophin modulators which overcome the limitations of ezutromid. Current efforts within the group are focused on optimising these promising new leads, as well as the use of chemoproteomic technologies to investigate compound mode of action and molecular target. Our overarching aim is to develop a pipeline of next generation molecules to deliver an effective therapy for all DMD patients.
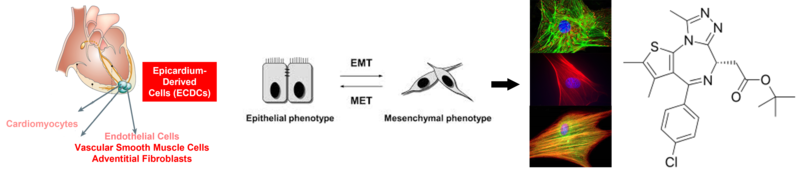
Small molecule activation of the adult epicardium for cardiac repair (with Paul Riley, Institute for Developmental and Regenerative Medicine)
The inability of the human heart to functionally repair itself in response to injury remains the biggest cause of morbidity and mortality in the global population. The most catastrophic injury, a myocardial infarction (MI), results in the instantaneous death of millions of cardiomyocytes that cannot be sufficiently regenerated by endogenous repair mechanisms.
Two cell types identified recently, based on their contributions to the developing heart during pregnancy, that can be activated during MI are cells derived from the outer epithelial layer of the heart, the epicardium, and the endothelial cells that make up the cardiac lymphatic vasculature.
An overview of state-of-the-art approaches to cardiovascular regeneration and repair post-MI will be provided. Our work aims to develop small molecules that can i) stimulate epicardium-derived progenitor cells (EPDCs) to produce new cardiomyocytes, and coronary vascular cells for therapeutic regeneration and ii) promote increased cardiac lymphatic vessel permeability and sprouting (lymphangiogenesis) to clear fluid build-up and traffic immune cells from the heart to reduce inflammation and improve heart function.
Small molecule-induced differentiation of cancer stem-like cells (with Paresh Vyas and Tom Milne, Weatherall Institute)
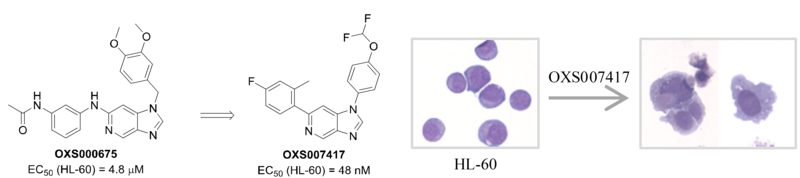
Acute myeloid leukemia (AML) is a type of blood cancer characterised by a block of differentiation, leading to the accumulation of immature cells in the bone marrow. Current treatments aim to kill these abnormal cells via chemotherapy.
We have developed a screening method and identified promising small molecules capable of differentiating Acute Myeloid Leukaemia (AML) blasts in vitro and used a chemoproteomics approach to demonstrate they act via a novel mechanism of action. We have progressed our most promising compounds into in vivo models of AML and shown they significantly reduced tumour growth and improve survival.
Anti-inflammatory agents (with David Greaves, Sir William Dunn School of Pathology)
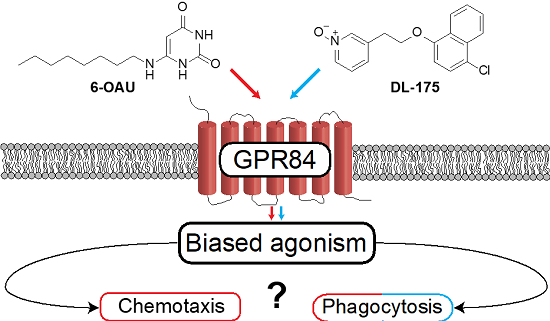
We demonstrated for the first time that a biased agonist of immunometabolic receptor GPR84, which activates Gai signalling but not b-arrestin recruitment (Dl-175), gave markedly distinct functional effects in primary murine and human macrophages from agonists acting via both pathways (6-OAU). The biased agonist was shown to prevent chemotaxis (immune cell migration) while stimulating phagocytosis (clearing of debris) in both mouse and human macrophages.
This is an important discovery of a small molecule along with a defined molecular target and cellular mechanism which is capable of blocking a pro-inflammatory response while stimulating a pre-repair response in both mouse and human systems and may have important applications in regeneration and repair. We are currently optimising the properties of this biased agonist and determining the structural basis of its functional activity.